Abstract
Background: In Australia, azacitidine is the only approved and subsidized hypomethylating agent for the treatment of patients with intermediate-2 / higher risk MDS and low blast count AML. There is paucity of real-world data in Australia on the impact of azacitidine on MDS progression and survival. This study retrospectively evaluated the use of azacitidine in Australia.
Method: Two observational cohort analyses were conducted for patients who had been prescribed Pharmaceutical Benefits Scheme (PBS)-listed azacitidine between 2016 and 2021 and for patients who had received azacitidine treatment between 2008 and 2015 at a tertiary hospital in Australia (Calvary Mater Newcastle, Australia). The number of treatment cycles, treatment persistence and overall survival (OS) as well as the impact of treatment adherence on OS were estimated where the data was available in each of the cohorts. Treatment persistence was defined as the time from the date of first azacitidine prescription until the date of last prescription of azacitidine prior to a gap of at least three months. OS was defined from the date of first azacitidine prescription to date of death.
The analysis was performed by Prospection Pty Ltd using the PBS 10% dataset. Provided data links for the 10% PBS cohort allowed for Kaplan-Meier survival analysis for patients initiating on azacitidine between January 2016 and April 2021.
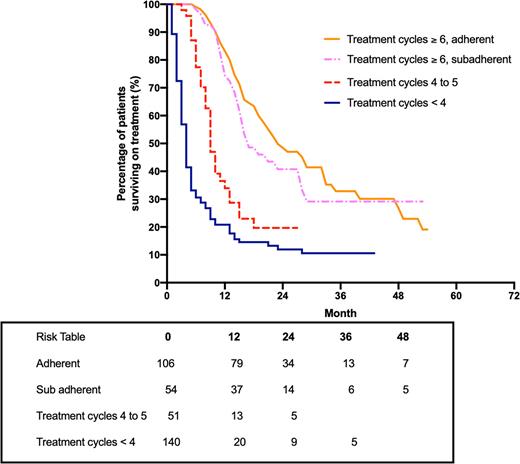
Results: There were 351 patients in the PBS 10% dataset who initiated treatment with azacitidine. The median persistence on azacitidine was 15.6 months and the median OS was 13.4 months. The median OS for patients who had six or more cycles of azacitidine treatment was greater compared to patients who had five or less cycles of treatment. The mean OS was 21 months (n=32) for patients who received azacitidine at the tertiary hospital and the mean time to acute myeloid leukemia (AML) transformation was 16.1 months.
Conclusions: This study illustrates the unmet medical needs for patients with MDS treated with azacitidine in Australia from two unique real-world data sets from different time periods. Most patients were not able to be treated with the optimal number of cycles of azacitidine which may have contributed negatively to their outcomes. The median OS on use of PBS listed azacitidine for MDS and low blast count AML in Australia is far inferior to that observed in clinical trials but similar to real world data from other parts of the world.
Figure 1: Kaplan-Meier estimate of overall survival for all patients with intermediate-2/HR-MDS, LB-AML or CMML who received six cycles or more of treatment, were adherent and sub-adherent to azacitidine (PBS 10% dataset). Overall survival data is also shown for patients who received treatment for either four to five cycles, or less than four cycles.
Disclosures
Enjeti:Otsuka Australia Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Caillet:Otsuka Australia Pharmaceuticals: Consultancy. Alam:Otsuka Australia Pharmaceuticals: Consultancy. Castaldi:Otsuka Australia Pharmaceuticals: Current Employment. Paine:Otsuka Australia Pharmaceuticals: Current Employment. Keer:Astex Pharmaceuticals, Inc.: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal